Our services | Industries | About us | Our locations | Our partners | Jobs

of the CSV tasks.

Dipl.-Ing. Thomas W. Höhle
Our services

Individual consulting
We accompany your organization on the way to secure CSV implementation: apt
- The regulated company is at the core to ensure patient safety, product quality and data integrity
- Management will commit to the requirements of the regulated environment.
- The employees will understand and implement the working world and the working methods of the regulated environment.
- The service providers will see themselves as an extended workbench of the regulated company and will act accordingly.
- The computerized systems and processes are validated exactly as it will be required based on risk and must be questioned with critical thinking.
- The work instructions and process descriptions are formulated precisely and are aimed at the actual regulatory purpose.
- Coordinated forms contain exactly the information required for the regulatory work environment.
- The process interfaces to the external and internal service providers are defined precisely.
- The scope of the documentation is managed according to the regulatory requirements.
- The service providers are accurately selected and qualified according to the regulatory purpose.
- The employees are appropriately prepared for the relevant audits.
- The internal CSV team is trained according to the requirements in a targeted and appropriate manner.
- The management receives appropriate rules for the control and traceability of the implemented measures.

In our online training portal CSVAcademy you will be trained in our modules in video lessons all about the CSV.
You can choose the place and time when and where you conduct the training. A video covers a lesson. You can decide for yourself how many lessons you want to watch in a row.
The lessons build on each other. Once you finish the current lesson, you will be unlocked for the next one. You can repeat the viewed lessons as often as you like.
After a thematic block, an appointment is made for a consulting session with one of our experienced CSV consultants. You can ask questions in this session about the current learning material or about CSV topics in your work environment.

Module 1: The world of CSV
- Introduction to CSV
- Regulatory requirements
- Implementation of the regulations with the help of norms, standards, guidelines, instructions and guides
- Part 11 & Annex 11
- 5 chapters
- 13 lessons
- 3 consulting sessions

Module 2: System Development Lifecycle
- The most extensive module for the complete guide GAMP® 5 in general
- and the 2nd edition in particular
- Detailed explanation of all 4 phases of the SDLC and their transitions:
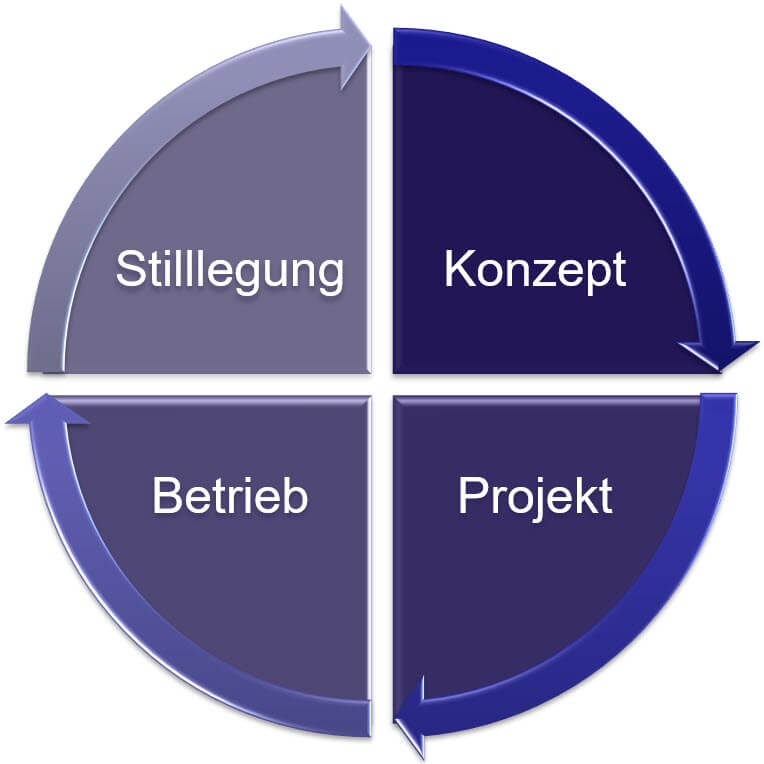
- 25 chapters
- 147 lessons
- 11 consulting sessions

Module 3: CSV Specialist
- Selection process to assess the knowledge of suppliers, subcontractors and service providers
- Establishing and maintaining relationships with suppliers, subcontractors and service providers
- Inclusion of suppliers, suppliers and service providers in your CSV processes
- Supplier Good Practices
- Contracts and their attachments such as SLAs and the QAA including suitable KPIs
- 5 chapters
- 19 lessons
- 3 consulting session

Module 4: CSV Auditor
- Audit forms
- Audits according to GAMP® 5
- Preparation, implementation and follow-up of audits
- Audit specifications according to norms and instructions such as the FDA
- 8 chapters
- 23 lessons
- 3 consulting sessions
Individual training packages
We are able to arrange tailormade packages or solutions for you from the modules, based on your knowledge and experience.
Visit our YouTube channel @CSV-Akademie or simply contact us directly:
Audits
We instruct suppliers and service providers and show them which requirements they have to fulfill in a regulated industry.

Industries
Pharmaceutical Industry
We train and consult online pharmaceutical customers regarding CSV and GxP based on GAMP® 5 - 2nd Edition. We help you to validate your production and business processes as well as the related systems.
Depending on the systems we coach and consult online your employees regarding the validation of applications and the qualification of IT infrastructures. Even though you outsource your IT Infrastructures to an IT provider i.e., cloud services.
We are glad to support your QA team by reviewing documents, maintaining your document management system (DMS) and creating standard operating procedures (SOPs), forms and templates.
Biotechnology
For example to bring together a LIMS (Laboratory Information System) with a MES (Manufacturing Execution System) and an enterprise resource planning (ERP) system to create an integrated network and ensure effective production in accordance with

We consult and support our customers
via internet regarding those topics and the verification of medical devices and their software. Thereby the IEC 62304 Edition 1.1 and if applicable the IEC 82304:2016 are the basis.
Moreover, we can assist you in considering related processes and systems regarding CSV at your company and the related documention.
About us
We are a motivated team of trained staff with many years of experience.
Thomas W. Höhle

The foundation of the engineering office CSV CSG - Computerized System Validation Consulting Services Group
Our locations
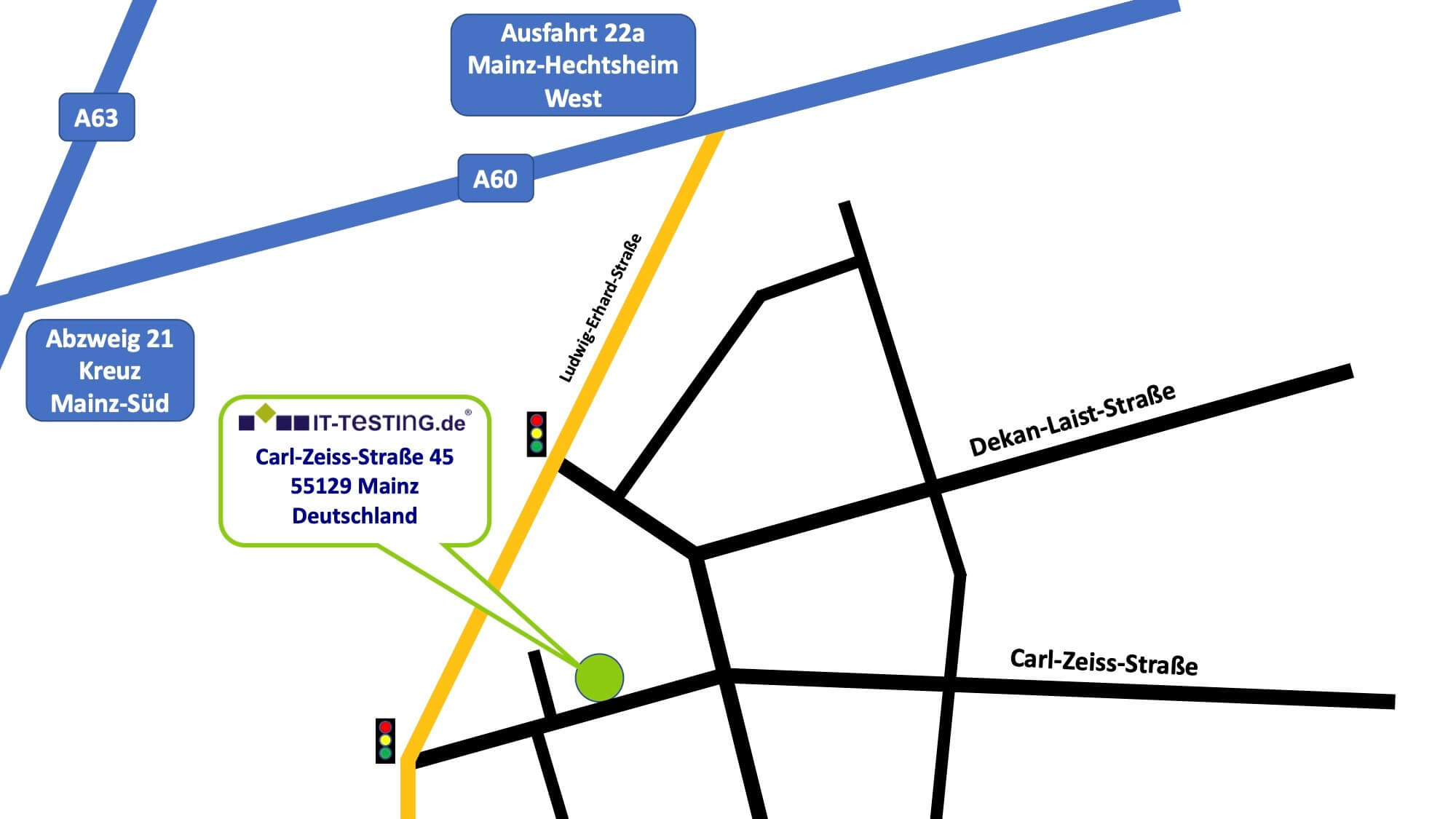

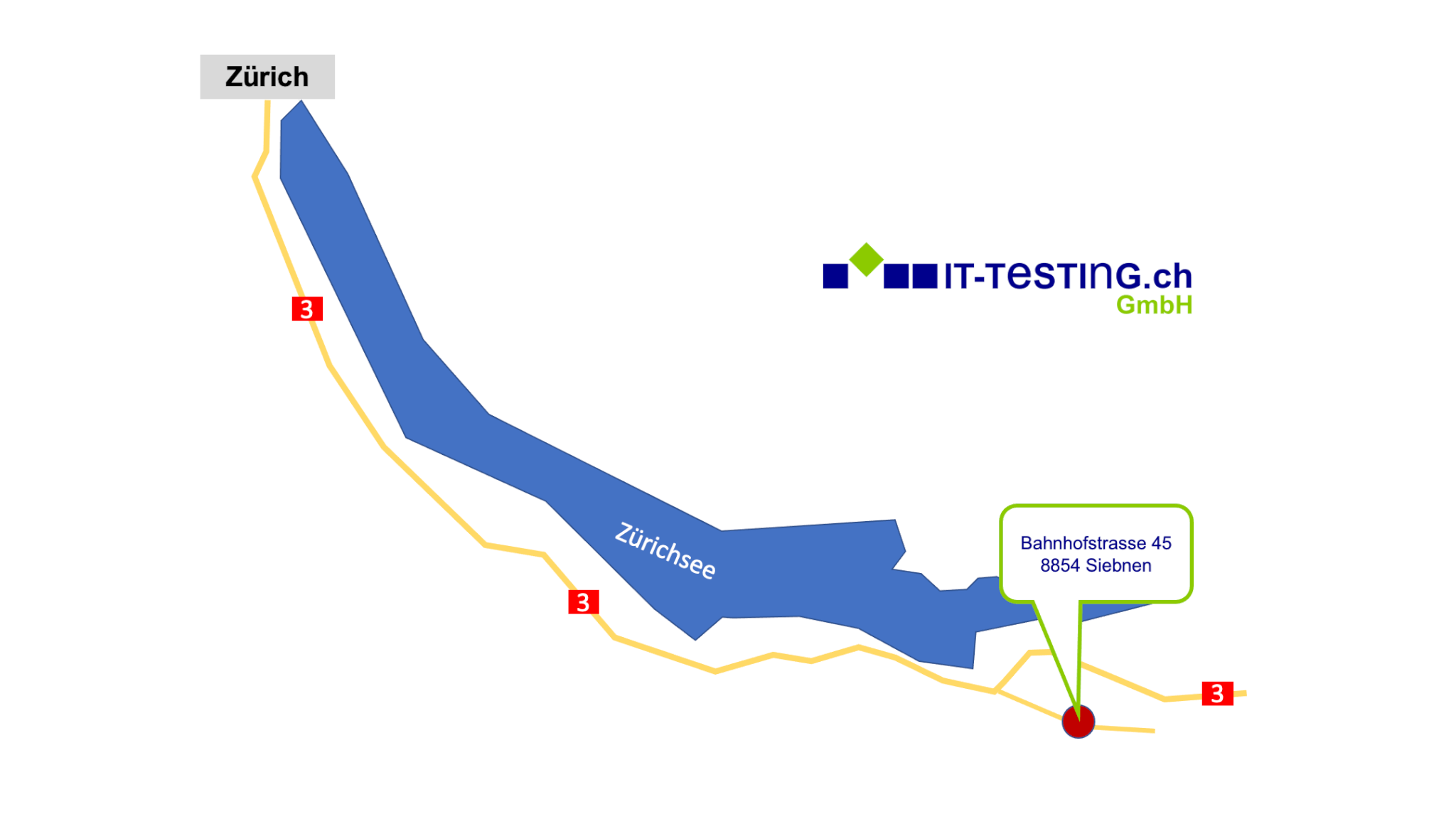

Our partners
BFMT Group
All from a single source.
"Everything from a single source" is an integral part of the corporate philosophy of the BFMT Group, they take care of you sustainably and are your partner in all corporate matters. BFMT offers you holistic services and solutions - compact and competent. The continuous further training of the experts of the BFMT Group has a high priority in the company culture. Additionally, the BFMT Group has a widespread network of experts.
The employees of the BFMT Group have extensive knowledge in the areas of auditing, tax consulting and management consulting. The experts of the BFMT Group have demonstrated this knowledge, among other things, through subject-specific personal certifications. The BFMT Group supports the engineering office IT-Testing.de® as a cooperation partner.QUAREGIA supports manufacturers of medical devices, in-vitro diagnostics, combination products and software in the areas of
- Quality management
- Software life cycle processes
- Cyber security
- Technical documentation
- Clinical evaluation / performance evaluation
- CE marking
- International product approvals
- Regulatory Affairs

Our ecological footprint
CSV Consulting Services Group is an Internet training and Internet consulting organization.We are aware of our particular responsibility for a clean environment. Therefore, at the end of the business year, we donate a part of our profit to projects and organizations to support tree planting.
CSVapt adapted precise tailormade CSV Computerized System Consulting Services Group CSVAkademie CSV-Akademie Validation Qualification Compliance QM Quality Management GxP Pharma Biotech Medtech Biopharma Medical Device Medical Device GMP GLP GCP GCLP GVP GAMP5 2. Edition GDP EC REP CH REP ISO 13485 14971 IEC 62304 82304 MDR MDD IVDR IVDD 9000 27000 27001 21 CFR Part 11 Annex 11 VoCS Computer Software Software Test Management SDLC Development Life Cycle Audit Inspection MDSAP QSIT FDA regulated Regulation regulating authority Notified Bodies IT Infrastructure Application Cloud Service Supplier Service Provider extended workbench Sustainability ecological footprint footprint